Our clinical program includes multiple achondroplasia clinical trials that are designed to better understand FGFR3-driven skeletal dysplasias and to evaluate an oral investigational therapy option for these conditions.

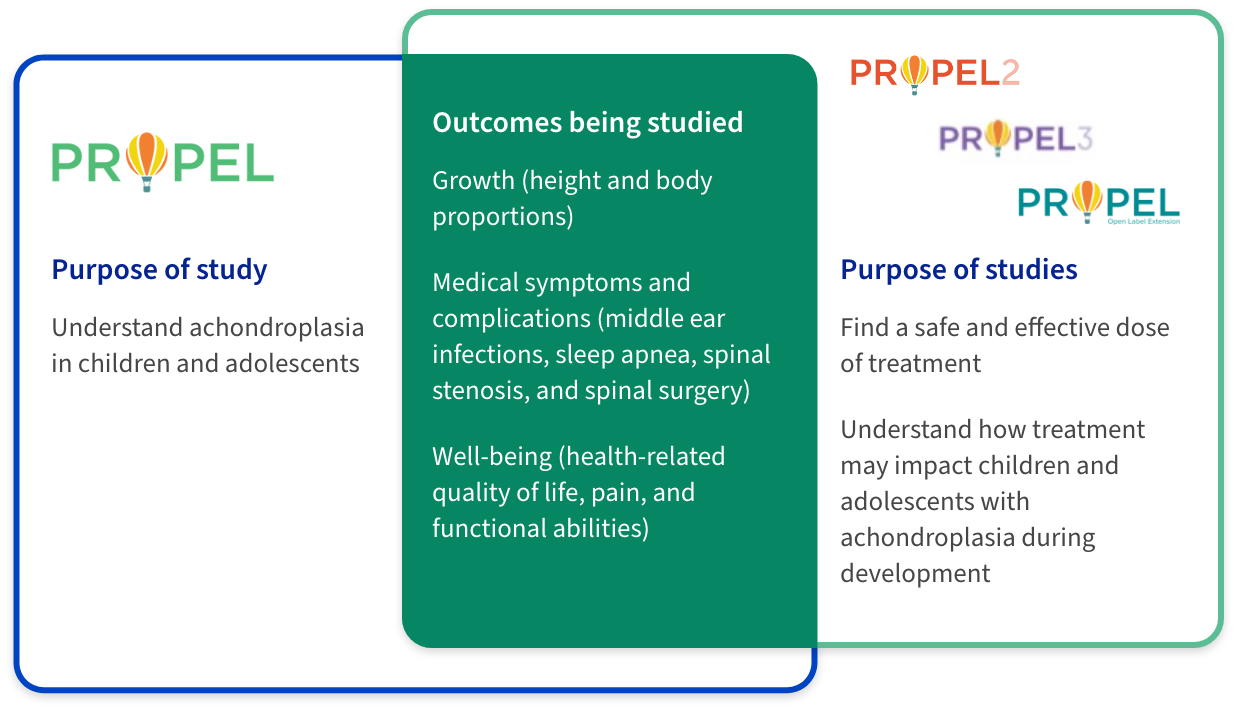
The PROPEL clinical program is studying a wide range of outcomes related to growth, medical challenges, and quality of life in children and adolescents with achondroplasia.
PROPEL is an observational study, in which no treatment is given. PROPEL 2 and PROPEL 3 are interventional studies, in which participants receive infigratinib and/or placebo.
A placebo is a treatment with no active properties, such as a sugar pill. Participants from PROPEL 2 and PROPEL 3 have the option to join the PROPEL open-label extension study, where they may continue to receive the investigational drug until their growth is complete.5-9
Participants who complete at least 6 months in the PROPEL observational study may enroll in PROPEL 3, the Phase 3 investigational study of oral infigratinib.9
Infigratinib is not currently approved for the treatment of achondroplasia by the U.S. Food and Drug Administration (FDA) or any other health authority.
PROPEL 3 is the next step in our process of developing a potential treatment option for FGFR3-driven skeletal dysplasias. PROPEL 3 is the first double-blinded study to compare the efficacy and safety of the oral investigational agent infigratinib with placebo in children and adolescents who have achondroplasia.9
Participants who complete at least 6 months in the PROPEL study may enroll in PROPEL 3. The primary goal of PROPEL 3 is to understand the effect of oral infigratinib on bone growth, as measured by annual height velocity (or AHV). This study will also look at the impact of the investigational treatment on well-being, pain, and functional abilities.9 Future studies will look at infigratinib in other FGFR3-driven conditions, such as hypochondroplasia.

For more details, visit clinicaltrials.gov
Visit clinicaltrials.gov