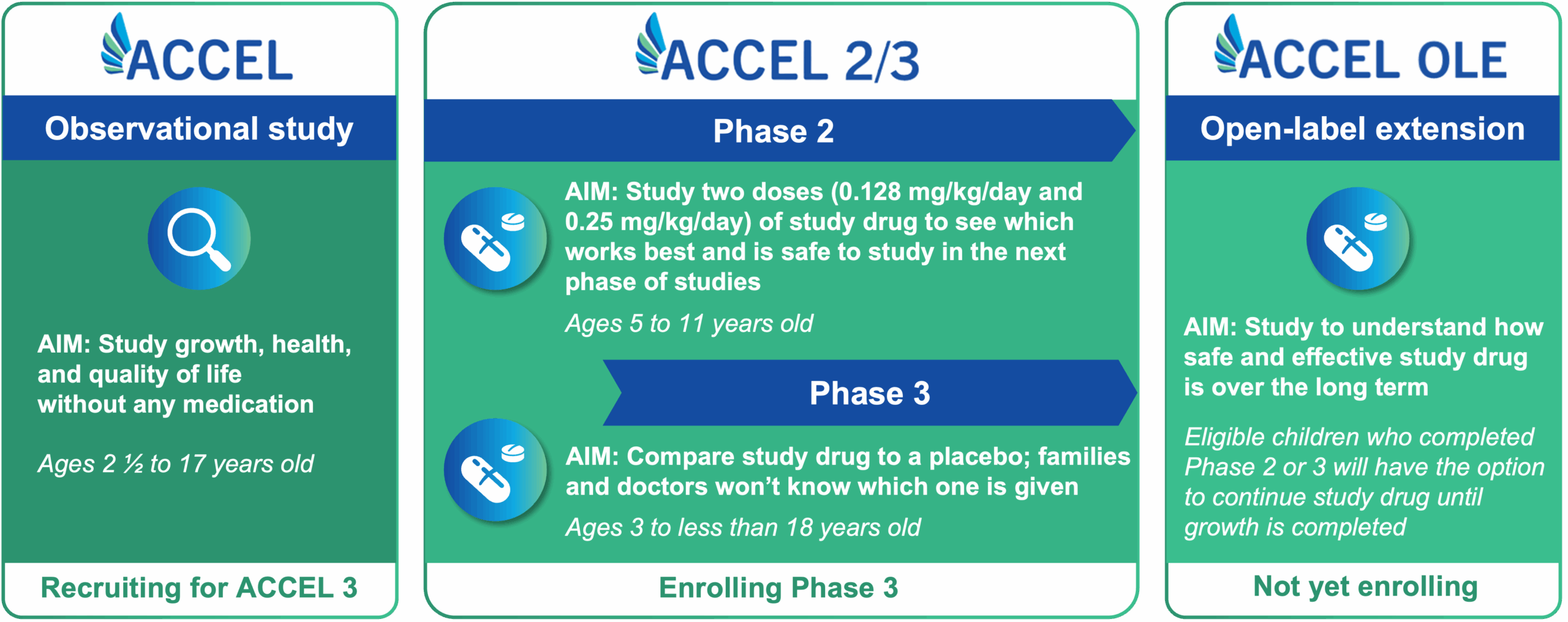
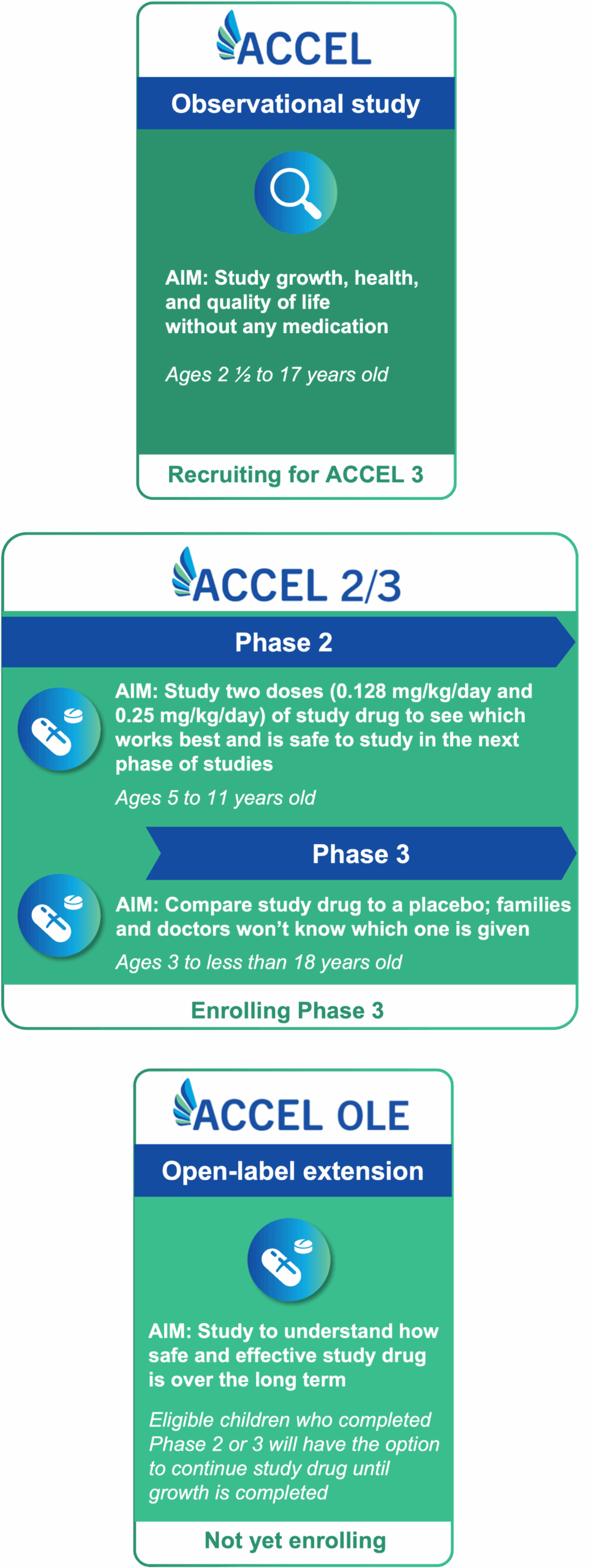
The ACCEL clinical program includes multiple studies grounded in learning more about hypochondroplasia and evaluating an oral treatment option for children and adolescents with this type of skeletal dysplasia.

The first phase of the ACCEL clinical program is an observational study. Children and adolescents participating in this phase will not receive any medication. Specific measurements will be taken by a healthcare provider over a period of up to 2 years.5
Participants are required to complete at least 6 months in the ACCEL observational study to be eligible to move on to Phase 2 or Phase 3, in which eligible participants may receive an oral medication called infigratinib. Infigratinib is being studied for its ability to help bones grow in children and adolescents with HCH.3
All eligible children and adolescents who participate in Phase 2 or Phase 3 will have the option to receive infigratinib at some point, including those who receive placebo in Phase 3.
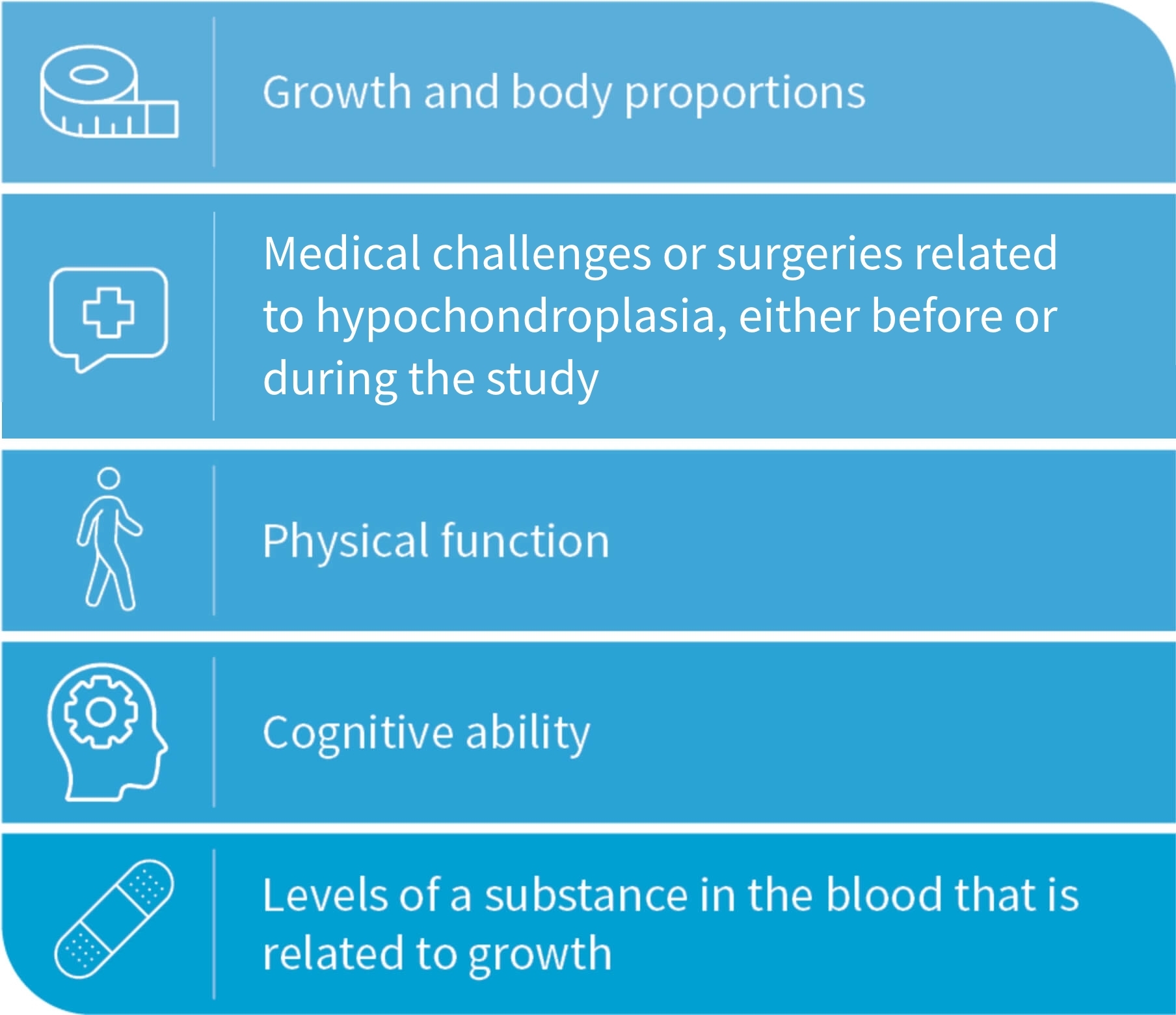
The measurements collected in ACCEL will lead to a better understanding of the growth, physical characteristics, thinking ability, and possible medical challenges that people with HCH can experience.
Children and adolescents must participate in the ACCEL observational trial for 6 months or longer to be eligible for participation in the Phase 2 or Phase 3 studies.